Igf-1 Stimulates The Growth Of Motor Neurons In The Brain
Video Link: https://vimeo.com/181528309
Video Download: Hgh Igf 1 Growth Factor Stimulates Rapid Extension Of Key Motor Neurons In Brain 2
Video Stream: Hgh Igf 1 Growth Factor Stimulates Rapid Extension Of Key Motor Neurons In Brain 2
Video Link: https://vimeo.com/181528307
Video Download: Hgh Igf 1 Growth Factor Stimulates Rapid Extension Of Key Motor Neurons In Brain 1
Video Stream: Hgh Igf 1 Growth Factor Stimulates Rapid Extension Of Key Motor Neurons In Brain 1
Video Link: https://vimeo.com/182905483
Video Download: Hgh Igf 1 Growth Factor Stimulates Rapid Extension Of Key Motor Neurons In Brain
Video Stream: Hgh Igf 1 Growth Factor Stimulates Rapid Extension Of Key Motor Neurons In Brain

IGF-1 stimulates Corticospinal Motor Neurons
In a new scientific study, a growth factor known as Insulin-like Growth Factor 1 (IGF) has been shown to encourage the rapid growth of corticospinal motor neurons (CSMN).
These neurons are vitally important because they connect the brain to the spinal cord, allowing the brain to interact and send signals throughout the body.
Although these neurons are necessary for the brain to control the human body, these cells are highly susceptible to diseases such as Lou Gehrig's disease, also known as amyotrophic lateral sclerosis.
What is ALS?
ALS is a disease in which the neurons in the corticospinal region of the brain and spinal cord atrophy and die. Over time, this leads to muscle tissue breakdown and neurological misfires by the brain. The muscles twitch as a result of incomplete connections. Eventually, ALS leads to a total breakdown of these neuron passageways, which inevitably completely paralyzes the arms and legs and eventually the torso.
ALS becomes fatal as the chest muscles lose contact and the patient can no longer breathe on his or her own. Strangely, ALS only affects motor movement and does not affect the brain or perception, although the effects on the body prove fatal over time.
IGF-1 Catalyses Axon Growth
In a recent publication of Nature Neuroscience, 2 researchers stationed at the Harvard Stem Cell Institute and the Massachusetts General Hospital have accurately described how IGF-1 leads to significant growth increases in the axons of corticospinal motor axons neurons in vitro testing.
Axons are long, thin strands that protrude from neurons, connecting them to other cells and allowing them to send messages via an electrical signal. When these axons atrophy, it reduces the ability of neurons to connect with one another and form sufficient circuits to deliver messages efficiently.
The Devastating Effects of CSMN Atrophy
Although in vitro testing is far from applicable medical theory, it is proof of concept. It shows how vital IGF-1 is to the human body and how future medical breakthroughs may be able to treat patients who suffer from illnesses that endanger CSMN cells.
Corticospinal motor axons are responsible for sending messages from the brain to the body to control the muscles. If there is a breakdown in these cells, 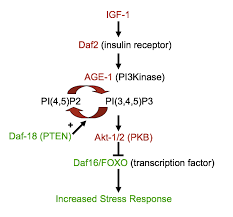 the brain will no longer be able to control the muscles, and paralysis and eventual death result.
the brain will no longer be able to control the muscles, and paralysis and eventual death result.
Researchers in this study found that IGF-1 is essential for the growth of CSM axons. If motor neurons are not provided sufficient IGF-1, their axons will not grow sufficiently in vitro. In addition, IGF-1 has been proven vitally crucial in motor neuron maintenance in animal research. When IGF-1 production was suspended in laboratory mice, it was found that Corticospinal motor axons did not grow as long or connect to adjacent cells as efficiently.
IGF-1 Proven Vital to CSMN Axon Growth
Jeffrey Macklis is the director of the Center for Nervous System Repair at Harvard Medical School. He is also the primary author of the study. He says that the research conducted by his team proves that IGF-1 can rapidly increase both the rate at which corticospinal motor axons grow and the length at which they grow.
Axons are well known for extending themselves long distances compared to the core cell. This study is the first evidence that directly shows that IGF-1 encourages the growth of corticospinal motor neurons.
Why is this Important?
Discovering this link is encouraging to Dr. Macklis, because it adds to the body of knowledge regarding how CSMN cells function and what stimuli help them develop. In addition, this knowledge may lead to future breakthroughs involving either IGF-1 or Human Growth Hormone, in which we may eventually be able to treat injuries of the spinal cord and neuron disorders such as ALS more effectively.
Today, the range of options is relatively small. Still, as the breadth of options increases with advances in medical knowledge, it is almost inevitable that new techniques will arise to provide improved outcomes for these patients.
What makes Corticospinal Motor Neurons unique?
CSMN cells are unique among neuron cells within the body. Their central bodies are located in the brain, but their axons reach all the way from the brain to their target neuron in the spinal cord.
Some axons extend all the way to the tip of the spinal cord, which in humans can be as long as three feet. Like most neurons of the brain and spinal cord, these cells have a minimal capability to reproduce. For this reason, they are incredibly at risk when severe spinal trauma or particular medical disorders destroy their tissue.
Damage to these cells directly leads to paralysis of the corresponding areas of the body, which a particular group of Corticospinal Neurons controls. Although these axons are incredibly long, they have been complicated to study until quite recently because of the vast number of other types of neurons occupying the same space within the cerebral cortex.
Hundreds of kinds of neurons are embedded within the tissue contained by the spinal cord. As a result, we do not know much about how CSMN cells grow and develop, although we are keenly aware of their function and the effects of their atrophy.
Isolating CSMN Cells for Study
Because of the difficulty of studying living Corticospinal Motor Neurons, Dr. Macklis and his post-doc research fellow Dr. Hande Ozdinler had to devise an innovative way to remove CSMN cells from their natural environment and isolate them in solitary populations in vitro.
Through a mixture of biological hypotheses and trial-and-error, Dr. Macklis discovered that IGF-1 was one of the primary candidates which seemed to facilitate the growth of CSMN cells.
By utilizing these isolated neurons, the duo proved in a laboratory environment that it was possible to encourage the growth of Corticospinal Motor Neurons with the application of IGF-1. They proved this in two ways.
One way that they achieved this growth was by directly applying IGF-1 to the purified cultures. The second way was applying IGF-1 to the purified 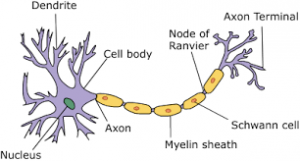 culture via microbeads coated in the hormone.
culture via microbeads coated in the hormone.
They found that CSMN axons grew by fifteen to twenty times in length as a direct result of IGF-1 stimulation. In a living system, this growth rate had only been observed during the initial growth of the axons.
When IGF-1 was removed from the system, CSMN axons again only grew at the pace they did during the control stage. This proves that IGF-1 is central to the complete development of the Corticospinal Motor Axons.
Several different experiments were conducted after they made the connection between IGF-1 and CSMN development. In these experiments, they tested the effect of IGF-1 on another neuron form and discovered that it had no appreciable effect.
They also tested other types of Human Growth Factor, applying them to CSMN cells to see if any other hormones could produce a similar effect. Only IGF-1 was able to increase the rate at which CSMN cells developed rapidly.
IGF-1 only affects the CSMN Axon
Dr. Macklis and Dr. Ozdinler also showed that IGF-1 did not play an active role in the survival mechanisms of the cell's core. It appears that IGF-1 only encourages the rapid lengthening of CSMN axons rather than having any influence on the central development of the cell.
The duo performed tests on living laboratory mice in which they blocked the pathway which routed IGF-1 to the spinal cord.
This alteration did not affect general cellular health but retarded the development of the CSMN axons. This animal test proved that the in vitro evidence could be applied to the biology of a living subject.
Understanding the Role of IGF-1 in Corticospinal Motor Cells
Dr. Macklis says that fully revealing IGF-1s role in developing Corticospinal Motor Axons is vital to fostering a complete understanding of how CSMN cells function and how we can prevent or cure spinal disorders and prevent CSMN Neurons from performing their necessary task of muscle control.
Although it may still be a long way off, studies such as these represent the initial steps in creating treatments that can alleviate degenerative disorders such as Lou Gehrig's disease.
In addition, if scientists can unlock the mechanism by which the hormone IGF-1 develops CSMN axons, we may one day be able to regenerate CSMN cells utilizing a mixture of IGF-1 therapy and neuronal stem cells.
Dr. Macklis research was fostered by grants from the Harvard Center for Neurodegeneration and Repair, the ALS Association, and the National Institutes of Health.
This information was collected from information released by Massachusetts General Hospital.
The New and Exciting World of Human Growth Hormone Research
We are just beginning to learn about the fascinating ways in which hormones such as Human Growth Hormone and IGF-1 maintain proper health and development. IGF-1 is an incredibly versatile hormone that is derived from Human Growth Hormone.
HGH is secreted by the pituitary gland and converted into IGF-1 in the liver. After converting into IGF-1, the hormone is distributed throughout the body to perform various tasks.
We have long known that IGF-1 directly leads to the breakdown of fatty tissue, enhancing weight loss and providing energy and nutrients required for muscle development. There are many other purposes of IGF-1 that we are just now discovering, and we will likely be learning more about its fantastic physiological benefits for decades to come.
muscle development. There are many other purposes of IGF-1 that we are just now discovering, and we will likely be learning more about its fantastic physiological benefits for decades to come.
IGF-1 plays a central role in anti-aging and long-term health maintenance due to its interaction with fat and muscle tissue. Over time and as a result of these studies, Hormone Replacement Therapy will become even more popular as the litany of benefits continues to be unearthed.
The future of IGF-1 Treatments
Dr. Macklis research shows that IGF-1 also plays a significant role in regenerative medicine. Eventually, IGF-1 will likely play a central role in genetic enhancement. This study is perfect proof of that. There is a significant chance that stem cells will be able to be repurposed as CSMN cells in the brain and spinal cord.
If surgical implantation is possible, IGF-1 will play a central role in restoring motor function in paralyzed patients. Imagine a world where the paralyzed can walk again. Imagine a world where diseases such as ALS are treatable rather than forces that can only be stopped, not slowed. This is the future of Anti-Aging Medicine, and HGH and IGF-1 will be critical players in the race to 150.
- 0001 Research Hgh For Patients Over 60 [Last Updated On: June 8th, 2025] [Originally Added On: September 30th, 2020]
- 0002 Natural Hormone Replacement Abc News 13 [Last Updated On: October 25th, 2025] [Originally Added On: October 2nd, 2020]
- 0003 My Own Experience With Human Growth Hormone Case Study [Last Updated On: February 10th, 2025] [Originally Added On: October 3rd, 2020]
- 0004 Ipamorelin Alternative To Hgh Injection Therapy [Last Updated On: February 10th, 2025] [Originally Added On: October 4th, 2020]
- 0005 Human Growth Hormone Therapy For Adult Hgh Deficiency Do The Benefits Stack Up [Last Updated On: February 2nd, 2025] [Originally Added On: October 5th, 2020]
- 0006 Human Growth Hormone Rehabilitation Benefits [Last Updated On: June 1st, 2025] [Originally Added On: October 6th, 2020]
- 0007 HRT [Last Updated On: May 5th, 2025] [Originally Added On: October 7th, 2020]
- 0008 Hormone Replacement Therapy With Pellet Implants [Last Updated On: May 4th, 2025] [Originally Added On: October 8th, 2020]
- 0009 Omnitrope Bio-identical HGH Injections [Last Updated On: February 2nd, 2025] [Originally Added On: October 9th, 2020]
- 0010 Imported Human Growth Hormone Is Illegal In The United States [Last Updated On: February 1st, 2025] [Originally Added On: October 10th, 2020]
- 0011 Estrogen Hormone Replacement Therapy May Diminish Alzheimers Disease Risk [Last Updated On: February 1st, 2025] [Originally Added On: October 12th, 2020]
- 0012 The Potential Benefits Of Estrogen On Alzheimer's Risk [Last Updated On: January 31st, 2025] [Originally Added On: October 13th, 2020]
- 0013 Dr. Hotze Talks About A Natural Solution For Depression. [Last Updated On: October 27th, 2025] [Originally Added On: October 14th, 2020]
- 0014 Anastrozole Research Study [Last Updated On: January 30th, 2025] [Originally Added On: October 15th, 2020]
- 0015 Symptoms Of Hormonal Imbalance In Women: What To Look For [Last Updated On: March 15th, 2025] [Originally Added On: May 12th, 2021]
- 0016 Beware of Fake Hormone Booster Pills – Sign Up for the Real Deal [Last Updated On: February 27th, 2025] [Originally Added On: December 21st, 2022]
- 0017 Chronic Fatigue versus Hormone Deficiency: How Can Patients and Doctors Tell the Difference? [Last Updated On: April 9th, 2025] [Originally Added On: January 18th, 2023]
- 0018 Ipamorelin: A Safer, Cost-Effective Alternative to HGH Therapy [Last Updated On: February 13th, 2025] [Originally Added On: February 13th, 2025]
- 0019 Treatment of Hormone Deficiencies [Last Updated On: February 15th, 2025] [Originally Added On: February 15th, 2025]
Word Count: 1750






