Serostim Bio-identical Hgh Injections
Hormone Replacement Therapy
SEROSTIM
Serostim Approved by the Food and Drug Administration as a Treatment for HIV-Related Cachexia
Serostim is the only Human Growth Hormone Medical Treatment approved by the FDA for treating muscle wasting associated with the HIV Virus. Serostim has been shown to increase muscle mass and stabilize and increase healthy weight while reducing fatigue and endurance for physical activity.
Serostim History
Seventeen years ago, Serostim was fast-tracked for approval by the Food and Drug Administration. Any drugs which offer new and unique treatment options for patients with a severe and critical ailment can qualify for this streamlined version of the FDA-Approval Process. After being placed on this approval track, Serono (the creator of Serostim Bio-Identical HGH) began a large-scale study to fully evaluate the usefulness and safety profile of Serostim HGH for HIV-Wasting.
This medical trial was double-blind in nature and controlled by a placebo. In addition to this, the large-scale trial also involved numerous independent 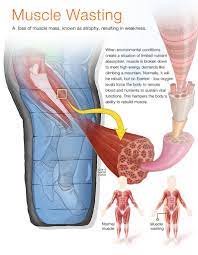 medical facilities. The results of this trial corroborated with initial small-scale trials, which hinted at the beneficial use of Serostim for this purpose.
medical facilities. The results of this trial corroborated with initial small-scale trials, which hinted at the beneficial use of Serostim for this purpose.
FDA-Facilitated HGH Research showed that Serostim could stimulate the production of lean muscle and significantly reduce fatigue in patients suffering from HIV-Related Wasting. In addition to quantitative benefits related to Serostim HGH, patients also felt that the benefits provided by Serostim were significantly qualitatively beneficial.
Muscle Atrophy Among Worst Symptoms of HIV and AIDS
Lean muscle wasting is one of the most detrimental symptoms of HIV, and Serostim has been proven to alleviate or reverse the effects of this devastating condition. Stronger muscles and decreased fatigue give the body more resources to fight off other symptoms associated with the HIV Virus, increasing overall health outcomes for those that suffer from HIV.
Although Antiretroviral medications can alleviate many of the debilitating symptoms of the HIV Virus, it is clear that Serostim provides an additional benefit that Antiretroviral Drugs alone cannot produce alone. Combining these HIV Drugs with Serostim allows patients to live healthier lives closer to normal.
Pitfalls of Cachexia Due to HIV
HIV-Related Wasting Disease is a disorder that becomes progressively worse over time without treatment. The adverse effects of lean muscle atrophy degrade the human body and rapidly increase mortality rates over time without treatment. HIV Wasting is a Metabolism Disease that leads the body to burn organs and muscle tissue for energy.
This process is a physiological response to harmful stimuli associated with the HIV-Virus.
Even though the human body still has stores of usable fat, the HIV Virus tricks the body into burning vital muscle tissue rather than fat tissue. Because of this, patients suffering from HIV-Related Cachexia have a viciously imbalanced body-fat percentage, as the body burns structural muscle rather than emergency fat.
Lean muscle is a blanket term that refers to a number of types of tissue throughout the body, including organ tissue, the muscles themselves, and blood cells within the cardiovascular system.
Decreased Lean Muscle Mass vastly increases the risk of extreme physiological exhaustion, illness, and infection, which can overwhelm the weakened immune system of an HIV patient and vastly decrease their life quality and their long-term odds of survival. Serostim was designated as an Orphan Drug Treatment for HIV-Related Muscle Wasting by the Food and Drug Administration in 1991.
HGH-AIDS Study Protocol
The final seal approval for Serostim Human Growth Hormone Replacement Therapy was granted as a result of a dose-variable, double-blind, randomized large-scale medical study funded directly to assess the benefits of Serostim HGH Treatments. This clinical trial included 757 participants who all suffered from Wasting due to HIV or AIDS.
Subjects were not limited to the United States; international medical facilities and patients also participated. Patients were either provided Serostim Growth Hormone Injections daily or were provided injections daily, half of which were Serostim, half of which were placebo. A third group was injected with a placebo daily and served as the control.
HGH-AIDS Research Results
All groups received injections for an identical period and were then physiologically assessed at the end of an initial treatment period. Researchers found that patients receiving daily HGH Injections had significantly increased levels of lean muscle compared to the other subset of patients who only received a half dose.
Both of these groups of patients benefited significantly compared to a third group that simply received placebo injections. In both experimental groups, patients could perceive the benefits of Serostim Subcutaneous Injections in their everyday life.
All patients who completed the first three months of the controlled clinical trial maintained treatment beyond the initial phase of a clinical study. Of the 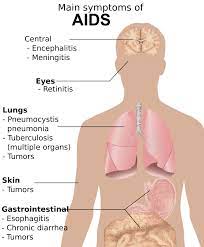 646 patients that completed the initial study, 548 remained in treatment for an extra three months. Among all of these patients, the benefits of Serostim on muscle mass maintenance continued to accrue.
646 patients that completed the initial study, 548 remained in treatment for an extra three months. Among all of these patients, the benefits of Serostim on muscle mass maintenance continued to accrue.
Currently, Serostim Recombinant HGH Therapy is the only HGH Treatment approved by the Food and Drug Administration for treating HIV-Related Cachexia. The dose recommended by the FDA is 0.1 Milligram per Kilogram each day.
For patients that weigh more than fifty-five kilograms, the maximum dose is six milligrams of Serostim per day. Patients at increased risk of side effects should be prescribed half of a standard weekly dose provided every other day.
HGH Caused Relatively Few Side-Effects
For the most part, Serostim produces very few side effects. The most reported side-effects of treatment were edema and non-severe joint and muscle pain. Any side effects are directly related to the size of the dose, and side effects eventually subsided either as treatment continued or with a reduction in dose size.
In a tiny minority of patients, issues related to glucose tolerance occurred, including the exacerbation of insulin sensitivity or the uncovering of underlying Type-Two Diabetes. In some of these latter patients, Insulin treatments had to be initiated or adjusted as a result of treatment. Patients with HIV-Wasting Disease with a history of Diabetes or insulin sensitivity should be closely monitored during HGH Treatment.
HGH High-Dosage AIDS Treatment
It should be noted that the Human Growth Hormone Dosage used to treat HIV-Related Cachexia is significantly higher than that used to treat Adult-Onset Human Growth Hormone Deficiency therapeutically.
Generally, the dose used to treat HGH Deficiency is between one and two IU per day, which is far less than the six milligrams per day generally prescribed to patients with severe Cachexia.
Human Growth Hormone has a remarkable capacity to stimulate the maintenance and development of lean muscle tissue in at-risk patients. In the future, there is no doubt that Human Growth Hormone Replacement Therapy will be highly beneficial for many other medical disorders.
- 0001 Smart And Simple Tips To Boosting Your Hgh Production [Last Updated On: August 9th, 2025] [Originally Added On: August 1st, 2020]
- 0002 Ten Tips For Naturally Enhancing Human Growth Hormone Production [Last Updated On: June 12th, 2025] [Originally Added On: August 4th, 2020]
- 0003 How To Safely Inject (HGH) Human Growth Hormone [Last Updated On: April 2nd, 2025] [Originally Added On: August 5th, 2020]
- 0004 Can Hgh Injections Help You Manage Your Weight More Effectively? [Last Updated On: June 11th, 2025] [Originally Added On: August 6th, 2020]
- 0005 Do Not Buy Hgh Sprays As Scams [Last Updated On: June 10th, 2025] [Originally Added On: August 8th, 2020]
- 0006 Can Human Growth Hormone Really Slow Down Aging? [Last Updated On: June 9th, 2025] [Originally Added On: August 9th, 2020]
- 0007 The Potential Side Effects Of Human Growth Hormone (HGH) [Last Updated On: February 16th, 2025] [Originally Added On: August 10th, 2020]
- 0008 Beware Hgh Scams: Only Use Bio-identical Growth Hormone [Last Updated On: April 22nd, 2025] [Originally Added On: August 13th, 2020]
- 0009 Human Growth Hormone Replacement Therapy For Hgh Deficiency [Last Updated On: September 11th, 2025] [Originally Added On: August 14th, 2020]
- 0010 How Does The Human Body Produce Human Growth Hormone? [Last Updated On: May 15th, 2025] [Originally Added On: August 17th, 2020]
- 0011 Do Not Buy Hgh Pills As Scams [Last Updated On: May 14th, 2025] [Originally Added On: August 18th, 2020]
- 0012 Hgh Injections From Mexico Are Dangerous And Illegal [Last Updated On: February 14th, 2025] [Originally Added On: August 19th, 2020]
- 0013 Human Growth Hormone Menopause Treatment [Last Updated On: February 20th, 2025] [Originally Added On: August 20th, 2020]
- 0014 Hormone Replacement Therapy Protects The Minds Of Hgh Deficient Patients [Last Updated On: May 12th, 2025] [Originally Added On: August 21st, 2020]
- 0015 Hgh Illegal Or Legal [Last Updated On: May 17th, 2025] [Originally Added On: August 22nd, 2020]
- 0016 How To Inject Hgh And Testosterone Safely And Easily [Last Updated On: May 13th, 2025] [Originally Added On: August 23rd, 2020]
- 0017 Human Growth Hormone Medical Research [Last Updated On: February 17th, 2025] [Originally Added On: August 24th, 2020]
- 0018 The Healing Properties Of Human Growth Hormone [Last Updated On: May 11th, 2025] [Originally Added On: August 25th, 2020]
- 0019 Hgh Therapy For Woman [Last Updated On: February 12th, 2025] [Originally Added On: August 26th, 2020]
- 0020 Hgh Therapy For Men [Last Updated On: April 19th, 2025] [Originally Added On: August 27th, 2020]
- 0021 How Does Age-related Hgh Decline Impact Health? [Last Updated On: February 17th, 2025] [Originally Added On: August 29th, 2020]
- 0022 Human Growth Hormone Injections Can Improve Joint Recovery After Injury [Last Updated On: May 10th, 2025] [Originally Added On: August 30th, 2020]
- 0023 The Anabolic And Bodybuilding Effects Of Bio-identical Hgh Injections [Last Updated On: May 9th, 2025] [Originally Added On: August 31st, 2020]
- 0024 Does Human Growth Hormone Really Have Healing Power? [Last Updated On: May 7th, 2025] [Originally Added On: September 1st, 2020]
- 0025 Getting HGH Growth Hormone Online [Last Updated On: May 8th, 2025] [Originally Added On: September 3rd, 2020]
- 0026 Buying Hgh [Last Updated On: June 4th, 2025] [Originally Added On: September 5th, 2020]
- 0027 Increase Your Potential With Hgh! [Last Updated On: June 3rd, 2025] [Originally Added On: September 6th, 2020]
- 0028 Can Bio-identical Hgh Help You Live The Life You Want? [Last Updated On: February 17th, 2025] [Originally Added On: September 8th, 2020]
- 0029 How Hgh Improves Libido [Last Updated On: February 17th, 2025] [Originally Added On: September 9th, 2020]
- 0030 Can Human Growth Hormone Speed Up Physical Rehabilitation [Last Updated On: September 17th, 2025] [Originally Added On: March 14th, 2021]
- 0031 A Beginner's Guide To Human Growth Hormone Replacement Therapy [Last Updated On: February 19th, 2025] [Originally Added On: March 16th, 2021]
- 0032 Can Hgh Injections Help You Live A Healthier And Happier Life? [Last Updated On: September 16th, 2025] [Originally Added On: March 19th, 2021]
- 0033 Buy Hgh Injections For An Introduction To Growth Hormone Therapy [Last Updated On: November 12th, 2022] [Originally Added On: March 20th, 2021]
- 0034 Improve Sexual Performance With Hgh Injections [Last Updated On: February 14th, 2025] [Originally Added On: May 12th, 2022]
- 0035 Understand The Risks Of Human Growth Hormone Overdose And Abuse [Last Updated On: October 15th, 2025] [Originally Added On: May 24th, 2022]
- 0036 Racing Ferraris – A Stressful Way to Earn a Living [Last Updated On: March 19th, 2025] [Originally Added On: July 12th, 2022]
- 0037 HGH: The Happiness Hormone [Last Updated On: March 26th, 2025] [Originally Added On: August 15th, 2022]
- 0038 Government, Contractor, and NGO Productivity, Efficiency, and HGH [Last Updated On: February 24th, 2025] [Originally Added On: September 7th, 2022]
- 0039 Without HGH, You Might as Well Be Dead [Last Updated On: March 11th, 2025] [Originally Added On: September 16th, 2022]
- 0040 Study Suggests Women Can Sniff Out Single Men [Last Updated On: April 23rd, 2025] [Originally Added On: February 17th, 2023]
- 0041 Geographic Distribution of HGH Deficiency [Last Updated On: May 3rd, 2025] [Originally Added On: July 13th, 2023]
- 0042 Sudden Onset of HGH Deficiency [Last Updated On: September 8th, 2025] [Originally Added On: September 22nd, 2023]
- 0043 The Emotional and Mental Health Effects of HGH Deficiency [Last Updated On: September 6th, 2025] [Originally Added On: September 28th, 2023]
- 0044 Understanding Hormone Replacement Therapy for Menopause [Last Updated On: February 6th, 2025] [Originally Added On: February 6th, 2025]
- 0045 Unveiling the Magic of Human Growth Hormone: A Colorful Spectrum in Medical Marvels [Last Updated On: February 16th, 2025] [Originally Added On: February 13th, 2025]
- 0046 Introduction: The Demand for Health-focused Care in Professional Environments [Last Updated On: February 15th, 2025] [Originally Added On: February 15th, 2025]
Word Count: 1100






